Enantioselective synthesis Warfarin
Keywords:
Enantiomeric one of two stereoisomers that aremirror images of each other that are "non-superposable"
Racemates: In chemistry, a racemic mixture, or racemate (
- to create aimed stereocenters.
4-Hydroxycoumarins 4-phenyl-3-buten-2-one
+
used as catalyst S,S-diphenylethylenedediamine(
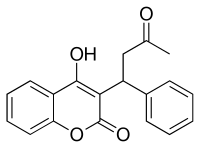
Warfarin product,
Proposed model by Terence C. Wong, Camille M. Sultana, and David A. Vosburg*
Reaction:
- Experimental Procedure:
1. 10 mL vial:
 - 0.162 g, 4-Hydroxycoumarins
- 0.162 g, 4-Hydroxycoumarins - 0.151 g, 4-phenyl-3-buten-2-one
- 0.021 g, S,S-diphenylethylenedediamine
- 2.0 mL THF(Tetrahydrofuran) THF
- 0.57 mL acetic acid
swirl the vial for a few min then keep in 1 week, stir is preferred to be used. the pink solutions turn to clearly yellow.
2. Check the progress of the reaction with TLC(CH2Cl2 on silitca gel)- using UV light to check warafin in the silica gel- the silica gel show the broaden band meaning that there the warfarin is mixed with a lot compounds. More procedures to be done to purify wafarin:
- remove the solvent and acetic acid by rotary evaporation or using a stream of air.
- residue is dissolved in a minimal amount of boiling acetone
- add boiling water dropwise ---solutions becomes significantly cloudy--->heat the mixture to dissolve the crystals.
- filter the solvent and collect the crystalline. waste the crystalline by ice-cold 4:1 acetone/water.(note that carefully handle the crystalline it may be dissolved to acetone and pass through membrane, and lost products will be considerable )
- check the product with TLC (take small amount of warfarin product then dilute in CH2Cl2 then put in the the silica gel, after running TLC, check by UV light),the result shows that warfarin is clean (shown 1 plot in the silica gel)
- measure the optical rotation of 10mg warfarin product in 6 mL 2-propanol using the polerimeter. = 0.032
- Calculate the enantiomeric excess of the isolated product.
Organic systhesis
Lab 1 organic synthesis
Lab 2 Purification of green fluourescent protein, GFP
Lab 3 Solid Phase peptide synthesis(SPPS) PLC
Fmoc strategy
Separation: Chromatography
Quantitative analysis
Mass spectroscopy: maldi-tof, ESI,
Inductively Coupled Plasma Mas Spectrometry
HPLC lab separation of peptides
Light spectroscopy
NMR spectroscopy
IR, X-ray spectroscopy, SEM
X-ray spectroscopy-diffraction
Lab 2 Purification of green fluourescent protein, GFP
Lab 3 Solid Phase peptide synthesis(SPPS) PLC
Fmoc strategy
Separation: Chromatography
Quantitative analysis
Mass spectroscopy: maldi-tof, ESI,
Inductively Coupled Plasma Mas Spectrometry
HPLC lab separation of peptides
Light spectroscopy
NMR spectroscopy
IR, X-ray spectroscopy, SEM
X-ray spectroscopy-diffraction









No comments:
Post a Comment
Bạn cần thêm thông tin hay có câu hỏi vui lòng comment